In 2018, the United States Pharmacopeia (USP) in General Chapters 1 and 2 updated the list of elements and maximum permissible daily exposure (PDE) values of elemental impurities in drugs, pharmaceutical substances, and raw materials. The USP is now aligned with the International Council for Harmonization (ICH) Q3D (R2) Step 5, the latest version of which was adopted in 2022.
Chapter specifies the list of elements and their maximum permissible daily exposure (PDE) values according to the modes of administration: oral, parenteral (intravenous injection) and inhalation drugs. Chapter details the sample preparation, analytical procedures, and quality control validation protocols for the analysis of elements specified in Chapter . The (ICH) Q3D (R2) Step 5 guidelines apply to the same group of pharma products and drug substances with the addition of limits for elemental impurities delivered via the cutaneous and transcutaneous route. It is a riskbased testing protocol for the assessment of the potential presence of toxic elements.
USP suggests adaptability of both ICP-OES and ICP-MS technologies for impurities analysis. However, the choice of technology depends on the permitted daily exposure (PDE), dosage forms and amount of daily dosage. ICP-OES has the sensitivity and linear dynamic range to handle some oral drug products; however, for the lowest limits of detection and widest linear range of calibrations, ICP-MS is the ideal technique.
In Table 1, the regulated elements and PDE limits from ICH Q3D are shown according to the routes of drug administration. They are divided in three classes (1, 2A/2B and 3), according to toxicity and likelihood of their occurrence in the drug products. For example, Class 1 and 2A, at a minimum, must be included in the risk assessment in all categories of drug products.
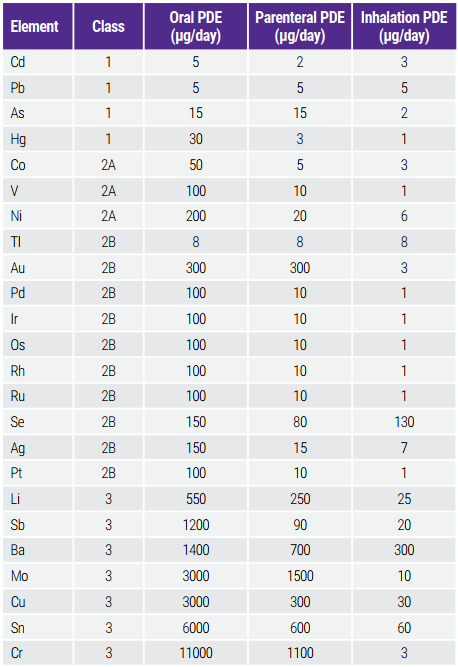
Bảng 1: Harmonized List of Elements and Established PDEs for Elemental Impurities
When the PDE values expressed in µg/day are changed to µg/g or µg/L based on a maximum daily dose, they are called Target Limits or Permitted Concentration. After a dilution factor is taken into account, they become J values that are calculated as follows:
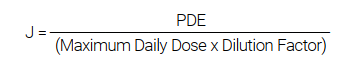
PerkinElmer offers a USP/ICH kit4 that, among other components, includes a J Calculator for accurate calculation of J values of elemental impurities, making standard preparation and method development much easier. This study focuses on the practical benefits of the NexION® 1100 ICP-MS5 for the determination of a group of toxicologically relevant elements in a large volume of parenteral (LVP) pharmaceutical products. It gives an overview of the USP methodology, with particular emphasis on the impurity levels and the recommended analytical procedures. Figures of merit for the system, based on the USP validation protocol for the method, are presented.
Experimental
Sample Preparation
A high-purity NaCl solution (seaBlank, 10-11% NaCl, Elemental Scientific Inc., Omaha, Nebraska, USA) was used to prepare the simulated large volume parenteral (LVP) saline solutions (0.9% NaCl) by an 11-fold dilution into a solution made of 2% HNO3 and 2% HCl. The simulated LVP saline solution thus prepared were further diluted 2-fold with the 2% HNO3 and 2% HCl solution into a final salinity of 0.45% before analysis. The USP requires the instrument calibration based on a minimum of two standards (0.5J and 1.5J). Pharmaceutical companies often use 0.3J as risk assessment. In extreme cases, if the sample contains an equal amount of J, samples spiked 1.5J will result in a concentration of 2.5J. Therefore, the low standard includes 0.3J and the high standard reaches 3J. Calibration standard concentrations are shown in Table 2.
The calibration standards were prepared by diluting commercial multi-element and single-element standards (see Consumables Used table) to concentrations based on PDEs, dilution factor of 2, and 2 L/day dose of saline solution samples. Internal standards consist of 1000 ppb Ge, 20 ppb Tb, and 20 ppb Bi in 2% HNO3 and 2% HCl and were mixed online with the blanks, standards and samples.
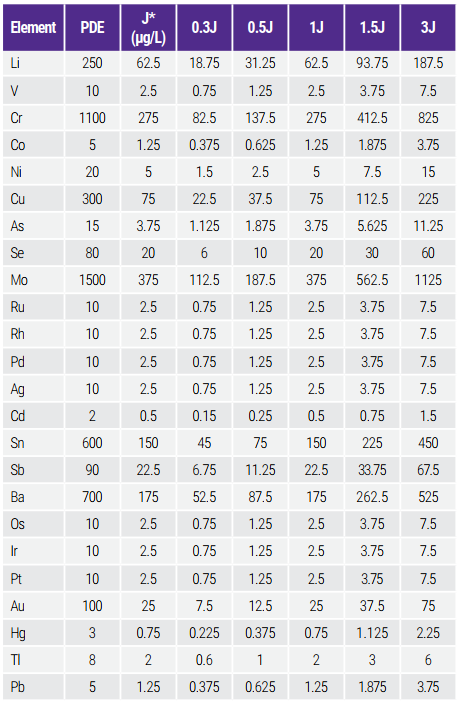
Table 2. PDEs (µg/day) and Calibration Standard Concentrations (µg/L) for LVP Saline Solution
Instrumental
A NexION 1100 ICP-MS (PerkinElmer, Shelton, Connecticut, USA) was used for the analysis of LVP saline samples in accordance with USP Chapters / and ICH Q3D. The NexION with patented ion optics design — the Triple Cone Interface-Quadrupole Ion Deflector combination and the quadrupole-based Universal Cell — combines the simplicity and convenience of a traditional collision cell with exceptional detection limits. In this application, helium was used as a collision gas for the measurement of all elements using Collision (KED) mode.
The cell gas flows were optimized for the best detection limits following the instrument operation manual.6 A default RPq (cell rejection parameter) was used for all the analytes to simplify method development. The elements, their isotopes, and helium (He) gas flows used for the analysis are listed in Table 3.
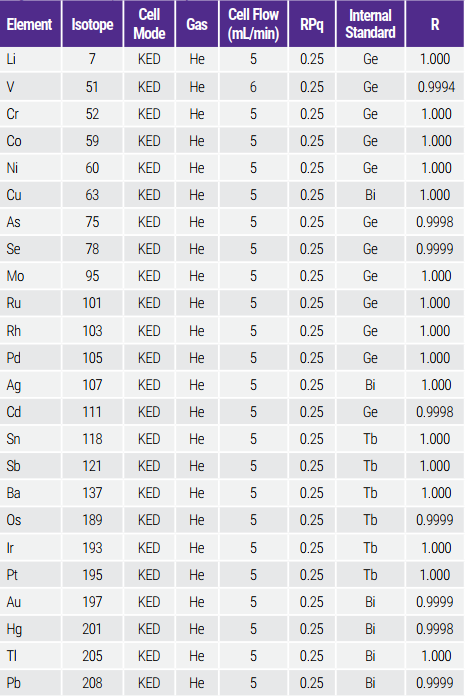
Bảng 3. Method Setup and Linearity
All Matrix Solution (AMS) with a flow of argon into the spray chamber neck, usually used for high-TDS samples, was applied here to stabilize signal, and afforded an additional 2-fold dilution.
Các thông số vận hành thiết bị được hiển thị trong Bảng 4. Trong chế độ va chạm (KED), tỷ lệ 35Cl16O/59The instrument operating parameters are shown in Table 4. In Collision (KED) mode, the ratio of 35Cl16O/59Co is typically 0.5%. For V analysis of a LVP saline sample, 35Cl16O+/59Co+ should be adjusted to less than 0.3%. Newly cleaned cones need to be conditioned prior to sample analysis. In this method, the cones were conditioned by aspirating 0.45% NaCl in 2% HNO3 and 2% HCl and monitoring the internal standards until the signals stabilized. The system was then washed by aspirating ultrapure water followed by 2% HNO3 and 2% HCl before starting the sample runs. 35Cl16O+ /59Co+ cần được điều chỉnh xuống dưới 0,3%. Các cone mới được làm sạch cần được điều chỉnh trước khi phân tích mẫu. Trong phương pháp này, các cone được điều chỉnh bằng cách hút 0,45% NaCl trong 2% HNO3 và 2% HCl và theo dõi các nội chuẩn cho đến khi tín hiệu ổn định. Hệ thống sau đó được rửa bằng cách hút nước siêu tinh khiết, sau đó là dung dịch 2% HNO3 và 2% HCl trước khi bắt đầu các lần chạy mẫu.
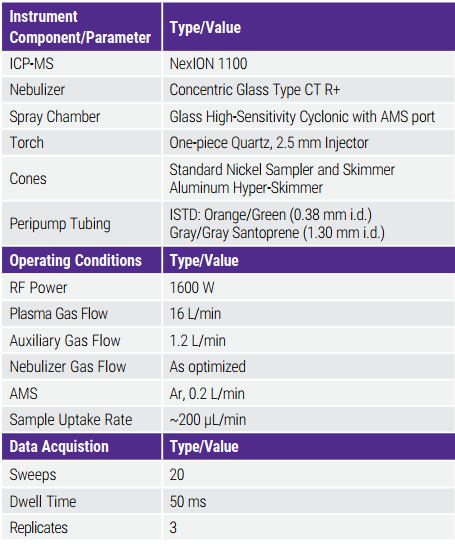
Bảng 4. Instrumental Parameters for LVP Saline Solution
Results
The USP 7 and define several QA/QC protocols to test and validate for method development. They include:
- Linearity
- Limits of detection – Detectability
- System suitability – Stability
- Accuracy
- Precision – Repeatability
Linearity
Validation criteria of the correlation coefficient should be greater than 0.995 for Category I assays and greater than 0.99 for Category II quantitative tests. Table 3 shows that correlation coefficients higher than 0.999 were achieved for all the analytes in the calibrated ranges.
Limits of Detection
The values of the method detection limits (MDLs) and method quantitation limits (MQLs) were established for all elements in this work to ensure that the detection capability of the method is substantially lower than the maximum elemental contaminant levels (1.0J). MDL was calculated as 3 * standard deviation (SD) of three replicated measurements of high-purity 0.45% NaCl (n=7) and MQL as 10 * SD of three replicated measurements of high-purity 0.45% NaCl. The results are shown in Table 5.
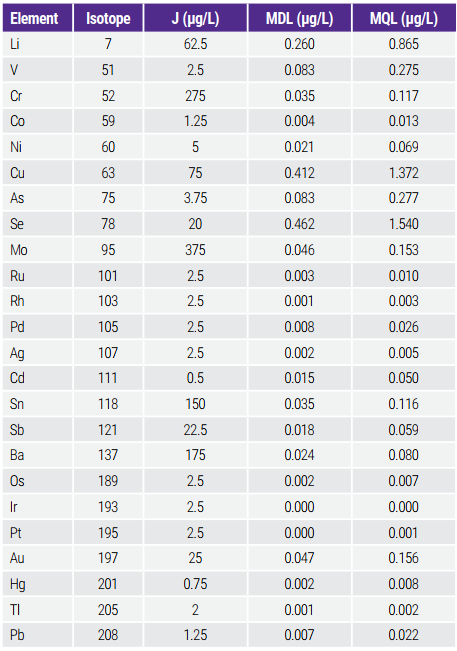
Bảng 5. MDLs and MQLs in Comparison with J
Detectability
For this test, the mean concentrations of the spiked samples with 1.0J and 0.8J, measured in triplicates, were compared. Per the acceptance criteria, the mean concentration (n=3) of the spiked samples at 1.0J must be within ± 15% of the 1J standard solution. The spiked samples at 0.8J must provide a signal intensity or value less than that of the 1J standard. The results presented in Table 6 show that these criteria were met/surpassed.
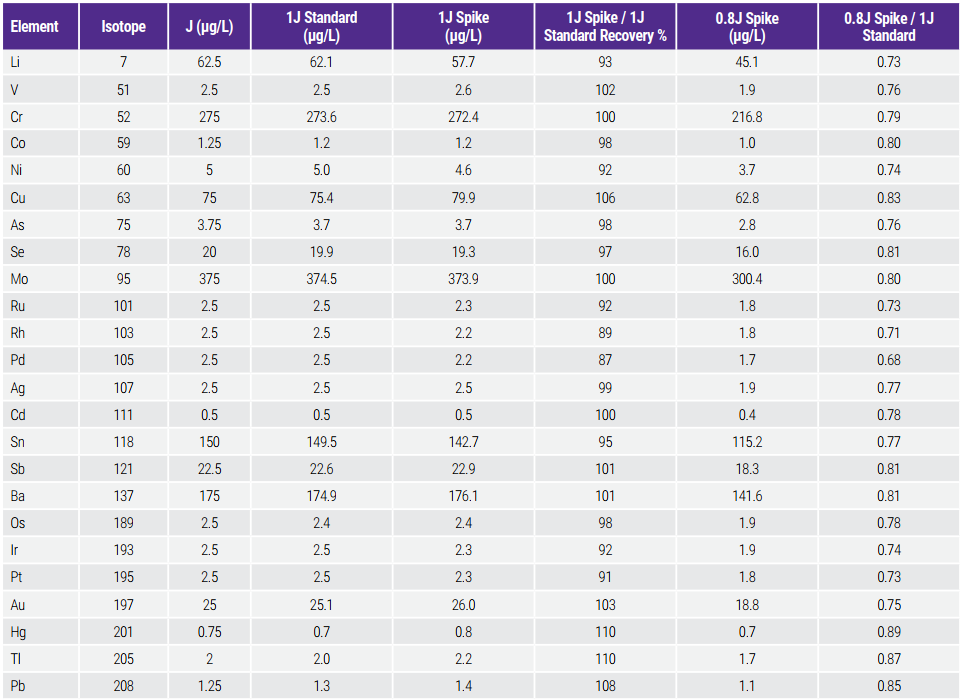
Table 6. Detectability Test Results
System Suitability (Stability/Drift)
This test requires comparison of results obtained from the 1.5J standard solution before and after the analysis of a batch of samples. The acceptance criterion requires not more than 20% drift for each target element. Table 7 shows the results for the 1.5J standard solution measured before and after the batch run of 45 saline samples (≈2 hours). The criteria were easily met with a drift of less than 6.3%.
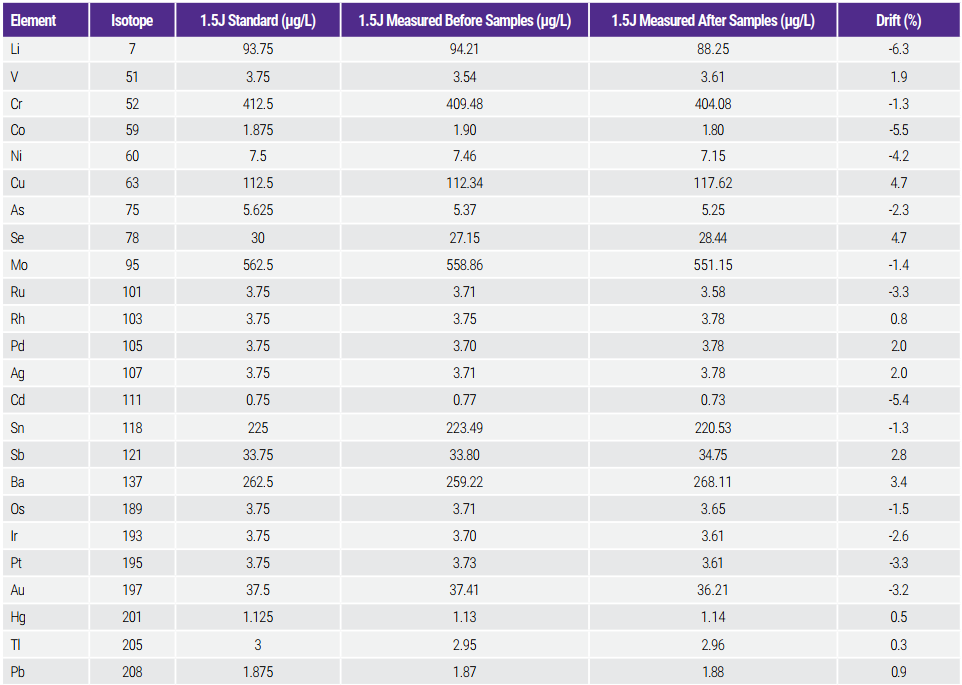
Table 7. Stability/Drift Test Results
Accuracy
Matrix spike recovery is used for the validation of accuracy. Per USP , appropriate standards at concentrations between 0.5J and 1.5J as well as corresponding spikes should be measured with the acceptance criteria of spike recovery between 70% - 150%. However, pharma companies usually adopt more stringent acceptance criteria by evaluating lower-level spikes to get better risk assessment. Therefore, spike recoveries at three levels (0.3J, 1.0J and 1.5J) were measured in this test. The results are shown in Table 8. Spike recoveries within 85% - 110% were achieved for all spike levels including the 0.3J spike. For all target elements, the relative standard deviation (RSD, n=3) was less than 10% for the 0.3J spike, and less than 5% for the 1.0J and 1.5J spike.
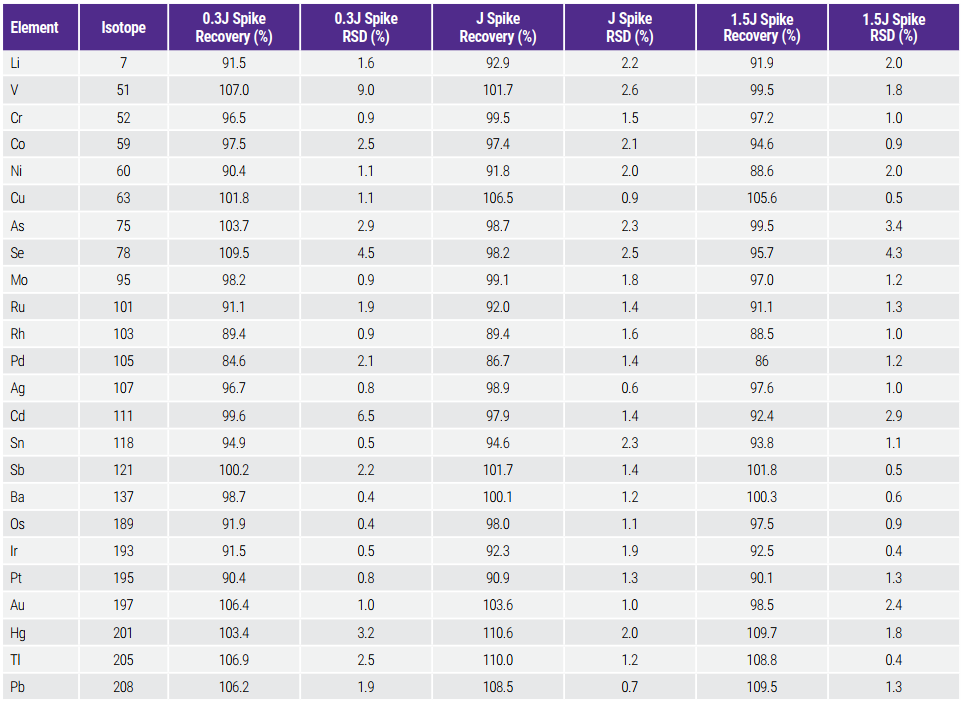
Bảng 8. Accuracy Assessment
Precision/Repeatability and Intermediate Precision
In the precision/repeatability test, the RSD of six samples spiked at 1J (target level) should be no more than 20% for each target element. The passing criterion for the Intermediate Precision test is the same; however, analysis of these six samples must be performed on different days, or with different instrumentation, or by different analysts. The criterion for RSD is equal to or less than 25%. As shown in Table 9, the RSDs are < 6.4% and < 6.6% for the precision and the intermediate precision, respectively, which are much lower than the specifications.
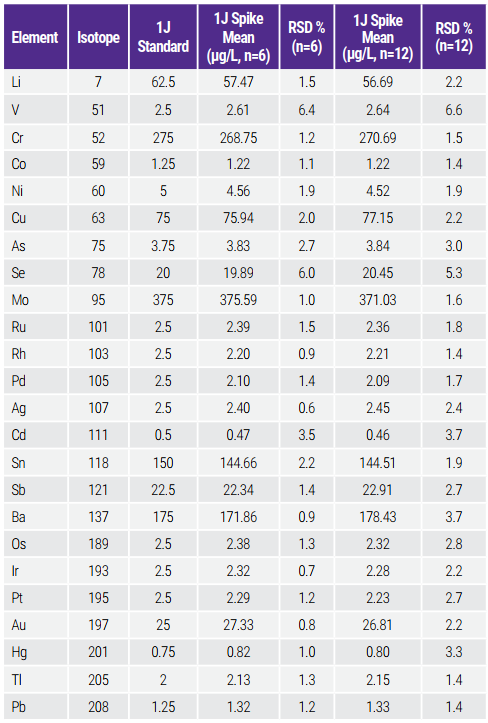
Table 9. Precision/Repeatability and Intermediate Precision Tests Results
Conclusion
Nghiên cứu này cho thấy NexION 1100 ICP-MS This study shows that the NexION 1100 ICP-MS working in the helium Collision (KED) mode is well suited for the analysis of pharmaceutical products according to USP Chapters / harmonized with ICH Q3D. The parenteral saline solution is one of the most challenging pharmaceutical products to analyze due to the large volume assumed, the high total dissolved solids (TDS) and the low PDEs. All 24 elements were identified and quantified by using helium Collision (KED) mode to remove the potential polyatomic interferences, and all validation requirements were met easily for linearity, limits of detection (detectability), system suitability (stability), accuracy and precision (repeatability and intermediate precision).
References
- United States Pharmacopeia General Chapter Elemental Impurities – Limits: USP 40 (First Supplement, 2017).
- United States Pharmacopeia General Chapter Elemental Impurities – Procedures: USP 38 (Second Supplement, 2015).
- ICH guideline Q3D (R2) on Elemental Impurities, Step 5, May 2022.
- USP / and ICH Q3D Toolkit, PerkinElmer, 2015.
- NexION 1100 ICP-MS Interactive Brochure, PerkinElmer, 2024.
- NexION Software Guide, PerkinElmer.
- United States Pharmacopeia General Chapter Plasma Spectrochemistry Method in USP National Formulary (NF).
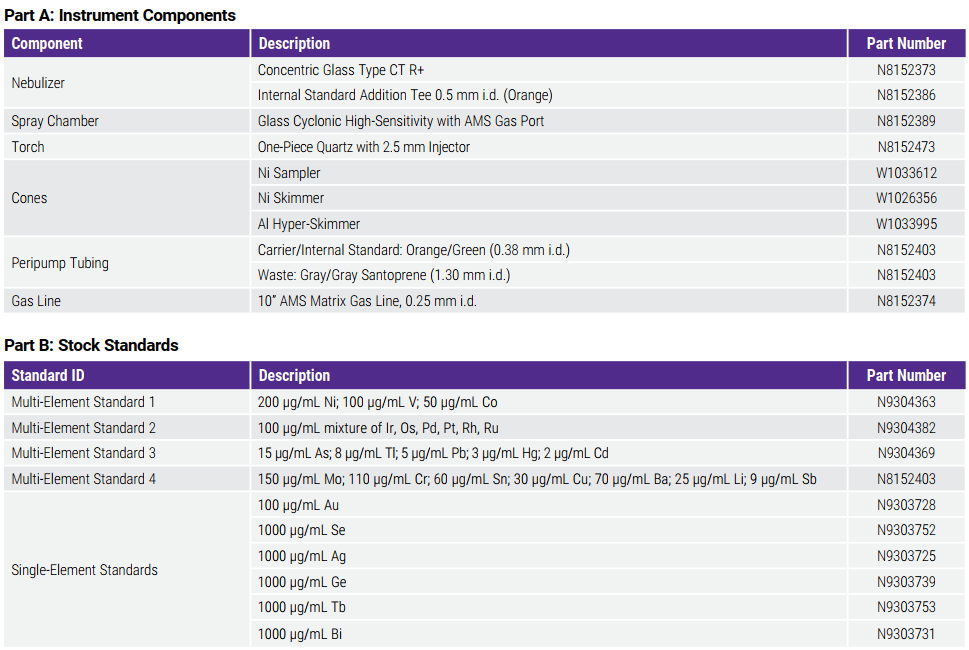
Consumables Used